Medical Physics Group
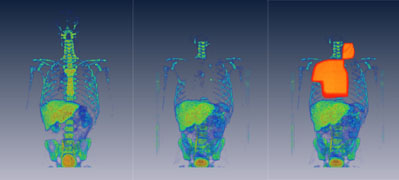 Figure 1: Series of first two images show the cellular
proliferation, imaged with FLT PET/CT before the
radiotherapy and after radiotherapy. Third image has
radiation field overlayed over the cellular proliferation
image. Series of images clearly show how the cellular
proliferation disappears in the irradiated bone marrow.
Figure 1: Series of first two images show the cellular
proliferation, imaged with FLT PET/CT before the
radiotherapy and after radiotherapy. Third image has
radiation field overlayed over the cellular proliferation
image. Series of images clearly show how the cellular
proliferation disappears in the irradiated bone marrow.
Medical Physics is an applied branch of physics concerned
with the application of the concepts and methods of physics
to the diagnosis and treatment of disease. Medical Physics
research in our group, strengthened with the research of our
collaborators at the Department of Medical Physics at
University of Wisconsin – Madison and our clinical
colleagues at the University of Wisconsin Carbone Cancer
Center, is focused into image-guided cancer* therapy.
Within this general area, our research is grouped into five
focused areas:
- First focus area is quantitative imaging of cancer,
with the aim to characterize biological properties of
tumors or to quantify treatment response, having
quantifiable imaging data becomes critically important.
Most of our research involves quantification of
molecular imaging techniques, predominantly positron
emission tomography (PET) and dynamic contrast enhanced
CT (DCE-CT).
- Second focus area is imaging for biological target
definition that will facilitate personalized treatment.
We are focusing on comprehensive definition of
biological targets that will be used for non-uniform
dose prescription - the process most often termed
"dose-painting".
- Third focus area is imaging for treatment assessment,
with the goal to characterize the tumor phenotypes
before the therapy and stratify the patients into
different treatment regimes. We are involved into a
series of Phase I clinical trials utilizing advanced
molecular imaging to determine early treatment response
and pharmacodynamics of several novel drugs,
particularly targeting angiogenesis.
- Fourth focus area is modeling of tumor growth and
response to therapies. Here, the goal is to test the
interplay of underlying biological processes, optimize
therapies, predict treatment response, and to generate
hypotheses in cancer research.
- Last focus area of our group covers open source
medical devices. The goal of the Open Source Medical
Devices (OSMD) initiative is to promote medical research
by making medical technology (hardware and software)
available as an open source to research groups around
the world.
* Cancer is the second (in some statistics even the first)
leading cause of death in the developed world, responsible
for over a third of all deaths, with an increasing incidence
from year to year. There are several ways of treatment,
depending mainly on the stage of the disease. Three main
cancer therapies are radiotherapy, surgery and chemotherapy.
Reference:
LIU, G., JERAJ, Robert, VANDERHOEK, M., PERLMAN, S.,
KOLESAR, Jill M., HARRISON, M.R., SIMOČIČ, Urban, EICKHOFF,
J.C., CARMICHAEL, L., CHAO, B., MARNOCHA, R., IVY, P.,
WILDING, G. Pharmacodynamic Study Using FLT PET/CT in
Patients with Renal Cell Cancer and Other Solid Malignancies
Treated with Sunitinib Malate. Clin Cancer Res, 17(24),
2011, 7 str., doi: 10.1158/1078-0432.CCR-11-1677.
[COBISS.SI-ID 25278759]
Beside head of the Medical Physics Group Dr. Robert Jeraj,
members of this group are
Dr. Urban
Simonèiè and
Damijan Valentinuzzi.
Dr. Urban Simončič working area:
Kinetic analysis of PET images – method optimization and
clinical applications
Positron emission tomography (PET) is already well
established imaging technique in modern oncology, but there
is still an unresolved problem of PET image quantification.
Quantitative measures can be extracted from PET data by
kinetic analysis methods or uptake normalizations. Kinetic
analysis is superior to the uptake normalization based PET
quantification methods in the richness and the specificity
of the results. However, kinetic analysis acceptance in
clinical settings is still limited due to the complexity of
data acquisition and its sensitivity to the imaging noise.
In order to address this issue, our work is focused into
improved kinetic analysis methodology comprising a robust
PET imaging protocol involving kinetic analysis that is
schematically presented below.
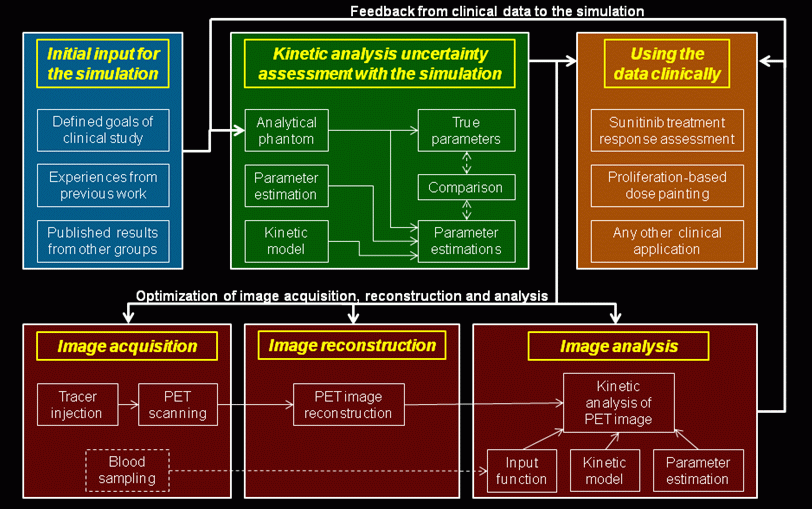 Figure 2:
The schematic representation of the robust PET imaging
protocol involving kinetic analysis. An overall output of
the robust PET imaging protocol involving kinetic
comprises the best possible kinetic parameter estimates
and a precise characterization of their uncertainties. To
obtain these estimates, concurrent optimization of the
kinetic analysis method, image acquisition method and
image reconstruction method is necessary. The optimization
procedure is iterative, with subsequent improvement of
simulation accuracy using the clinical data.
Figure 2:
The schematic representation of the robust PET imaging
protocol involving kinetic analysis. An overall output of
the robust PET imaging protocol involving kinetic
comprises the best possible kinetic parameter estimates
and a precise characterization of their uncertainties. To
obtain these estimates, concurrent optimization of the
kinetic analysis method, image acquisition method and
image reconstruction method is necessary. The optimization
procedure is iterative, with subsequent improvement of
simulation accuracy using the clinical data.
The developed methodology is then utilized in clinical
settings. There are two main types of clinical applications
for molecular imaging and kinetic analysis: 1) using the
imaging data for individual’s treatment assessment and
guidance, 2) using the imaging data in clinical studies with
the aim of discovering population behavior during the
treatment.
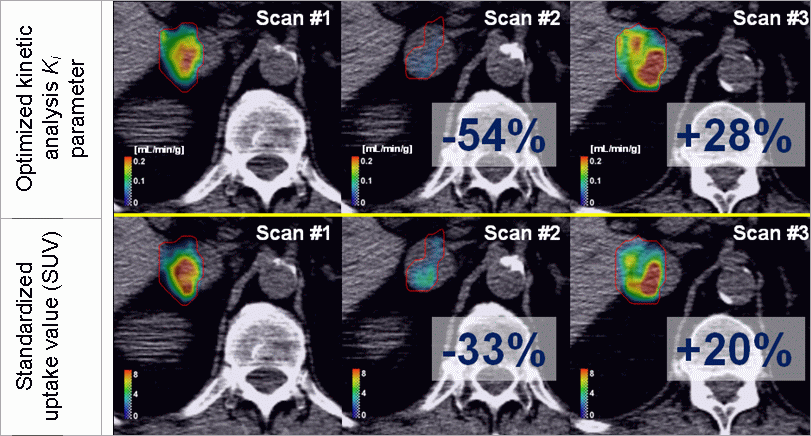 Figure 3: Here is an example of FLT PET/CT treatment
response assessment for the patients treated with
sunitinib malate. Upper row shows the treatment response
quantification with the optimized kinetic analysis, while
the lower image shows response quantification with a
standardized uptake value. The example shows that
optimized kinetic analysis produces considerably different
values for treatment response than the SUV. Superiority of
optimized kinetic analysis (in term of better accuracy)
can be tested with simulations.
Figure 3: Here is an example of FLT PET/CT treatment
response assessment for the patients treated with
sunitinib malate. Upper row shows the treatment response
quantification with the optimized kinetic analysis, while
the lower image shows response quantification with a
standardized uptake value. The example shows that
optimized kinetic analysis produces considerably different
values for treatment response than the SUV. Superiority of
optimized kinetic analysis (in term of better accuracy)
can be tested with simulations.
Preliminary results assure the improvement in individual’s
treatment assessment accuracy and the feasibility of more
aggressive treatment interventions. However, estimation
accuracy for population observables in clinical studies
could be limited with the clinical data heterogeneity and
lowering the individual’s treatment response uncertainty
does not necessarily affect the population response
uncertainty.
Damijan Valentinuzzi working area
One of the promising and emerging methods of treatment is
targeted drug therapy, which affects the specific processes
in the tumor. When tumor grows more than 1-2mm in diameter,
new blood vessels are required to deliver nutrients and
oxygen and they serve as an escape route for metastatic
cells. Our aim is modeling the tumor response to
anti-angiogenic drugs, which inhibit the growth of new blood
vessels. In particular, we are going to develop a computer
simulation, which will treat every patient and every single
tumor individually as unicum and by which it will be
possible to select the optimal therapy, that will give the
best results for each individual.
Currenty, we are investigating a multiscale computer model,
which combines experimental data from clinical studies on a
larger population of patients with data, specific for each
patient, which we obtained by using advanced imaging
techniques (PET/CT), capable of determining the heterogenity
within the tumor (proliferation, hypoxia, metabolism). We
believe that the lack of knowledge of each individual
tumor's heterogenity is an important reasons, why some
treatments do not end up as we expected.
Axitinib (Pfizer) is an example of an anti-angiogenic
targeted drug, which is still undergoing the clinical
trials, and which we would like to include in our
simulation. The drug inhibitsthe so-called VEGF TKI
receptors ("vascular endothelial growth factor - tyrosine
kinase receptors"), which are responsible for stimulating
the growth of new tumor blood vessels. We hope that by using
our model, we will be able to determine the optimal dosage
for each patient, predict clinical outcome and potential
interactions when using Axitinb in combination therapies,
for example in combination with radio- or chemotherapy.
Finally, we would also like to test different hypotheses as
to why sooner or later most tumors become resistive to
anti-angiogenic drugs, which is currently one of the main
problems.
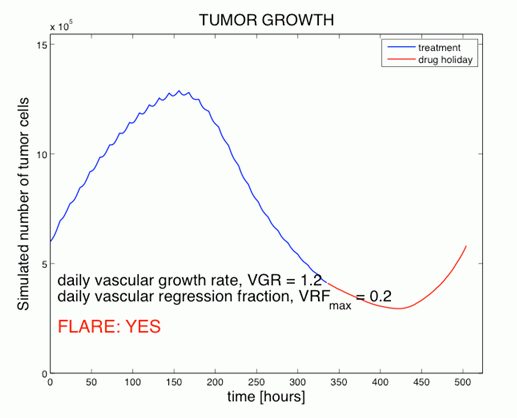
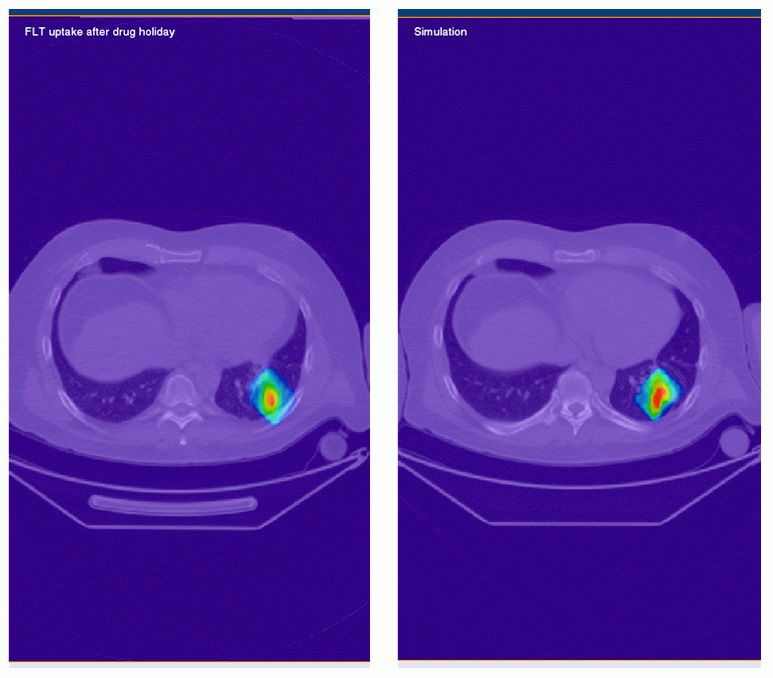 Figure 4 (above): Simulation of the number of tumor cells
during anti-angiogenic therapy (blue) and during one-week
drug holiday (red).
Figure 4 (above): Simulation of the number of tumor cells
during anti-angiogenic therapy (blue) and during one-week
drug holiday (red).
(below left): - real FLT PET image of cellular
proliferation in the tumor after one-week drug holiday.
(below right): - simulated FLT PET image of cellular
proliferation in the tumor after one-week drug holiday.









